The new Medical Device Regulation(EU) 2017/745 has been in force since May 2017. It brings significant impact to medical device industry and the human beings, not only for Europe market, but also for global markets. If you are an economic operator in the MDR, it is essential to know about the core and main improvement of the Regulation (EU) 2017/745 compared to Directive 93/42/EEC.
Objectives to Launch MDR
To reinforce the regulatory framework for medical devices, the European Parliament and the Council adopted the Regulation (EU) 2017/745, also known as the EU Medical Device Regulation, from May 2017. Patients and users are served with higher level of health protection. The functioning of the healthcare is therefore optimized in light of issues and weakness identified with Directive 93/42/EEC.
New Conformity Assessment System of MDR
Compared to the MDD, the MDR set out a stricter system of conformity assessment to medical devices placed on the EU market and introduces a life-cycle approach to ongoing CE Marking compliance from the manufacture, sale, distribution to the retirement of medical devices. The patient-centered healthcare improvement includes requirement on more robust technical documentations, strengthen of product safety and performance by post-market surveillance, as well as a transparent EU database on medical devices (EUDAMED).
.jpg)
Challenges to Apply for MDR
To implement the MDR compliance, there are main challenges well known for medical professionals. For instance, the growing backlog during the short transition period, the lack of process agility due to critical re-assessment to place the medical devices on the market, and higher pressure on scarce notified bodies and competent authorities.
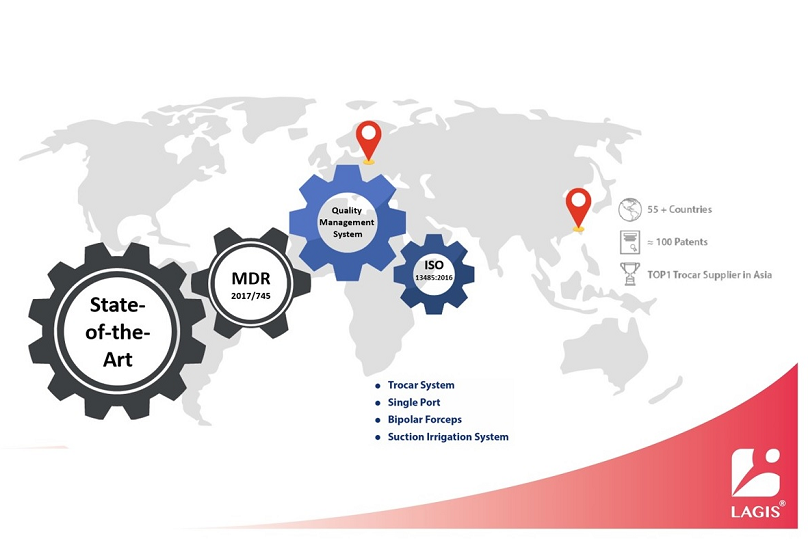
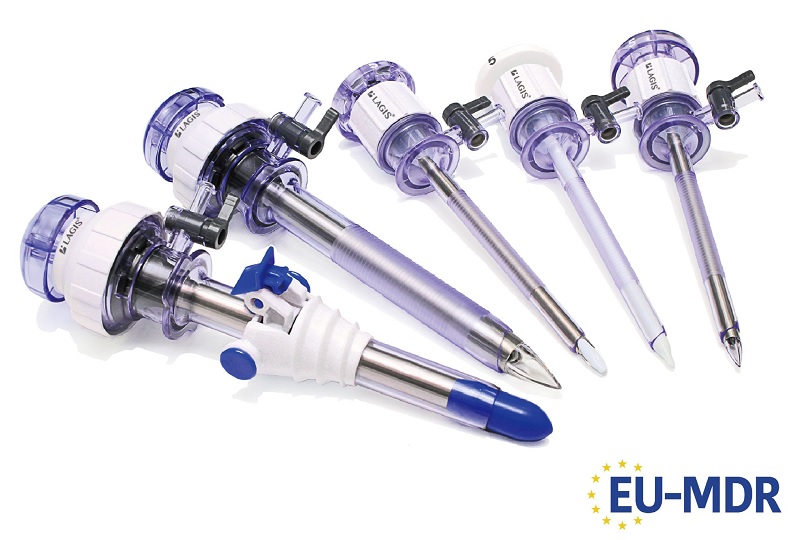
LAGIS has received CE (MDR) certificate in May 2022, and marketed trocars, single ports, suction irrigations, instruments, specimen retrieval bags, and wound retractors retractors, etc to Europe market. Please feel free to contact us for further product and cooperation information. Afterwards, we will sequentially list additional relevant information in the "Certification Info" section on the official LAGIS website.

We use cookies to provide the services and features offered on our website, and to improve our user experience. By using this website, you consent to the use of cookies.